1. What Happened?
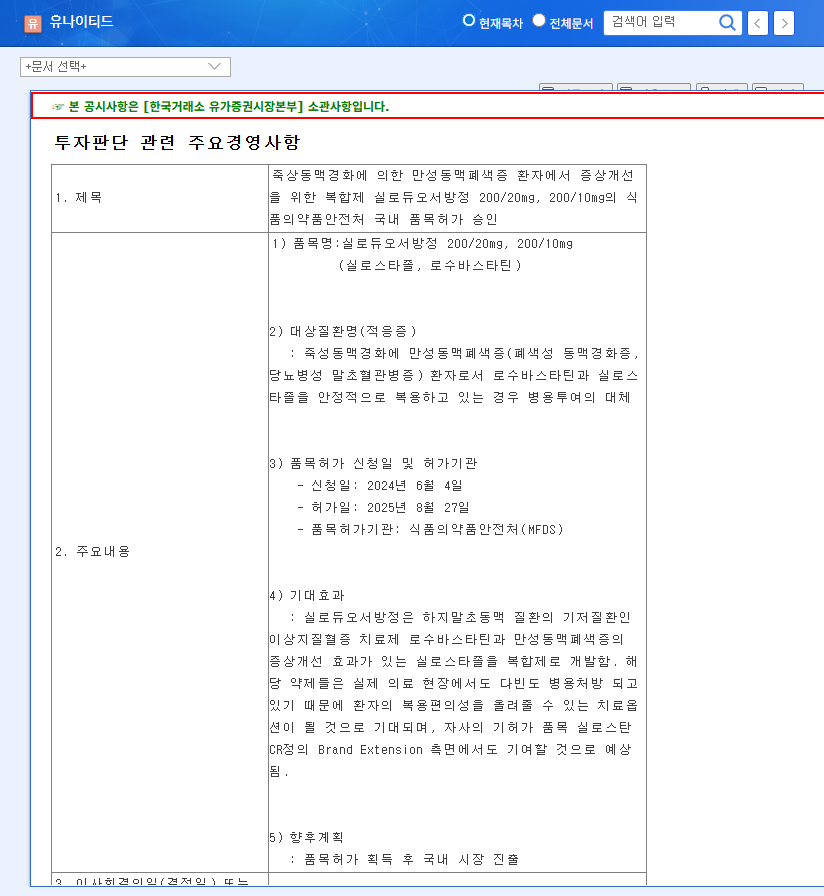
Korea United Pharm received domestic approval for Siloduo SR, a combination drug for chronic arterial occlusive disease, containing both cilostazol and rosuvastatin. This is expected to improve patient convenience and contribute to the expansion of the company’s existing product brand.
2. Why is it Important?
This approval signifies more than just a new product launch. It addresses the needs of the medical field by combining two frequently co-prescribed ingredients and reaffirms Korea United Pharm’s capabilities in developing modified new drugs. This creates the potential for increased market share and revenue growth.
3. What’s the Impact?
Positive Aspects:
- – Securing a new growth engine and expanding business portfolio
- – Increased patient convenience and potential for market expansion
- – Expected synergy with existing products
Potential Risks:
- – Time lag until actual sales generation and required marketing efforts
- – Potential for increased competition in the combination drug market
- – External factors such as pending litigation and drug price reduction policies
4. What Should Investors Do?
While this approval is a positive momentum, potential risks exist. Investors should closely monitor the following:
- – Market response to the new combination drug and actual sales performance
- – Progress and outcome of the lawsuit
- – Changes in the competitive landscape and government drug pricing policies
It is crucial to make investment decisions based on a comprehensive consideration of fundamentals and risk factors, rather than being swayed by short-term stock price fluctuations.
What condition is Siloduo SR used to treat?
Siloduo SR is used to treat patients with chronic arterial occlusive disease caused by arteriosclerosis obliterans.
Will this approval positively impact Korea United Pharm’s stock price?
It is expected to serve as positive momentum, supporting long-term growth potential, but short-term stock price fluctuations can vary depending on market conditions and various factors.
What are the key considerations for investment?
Closely monitor market response to the new product, actual sales performance, litigation risks, and be mindful of changes in the external environment.