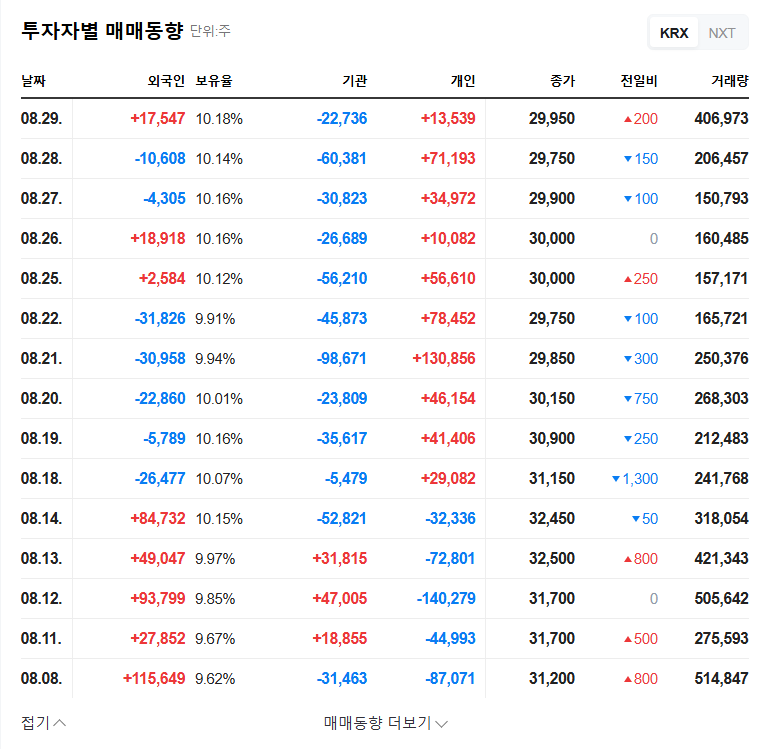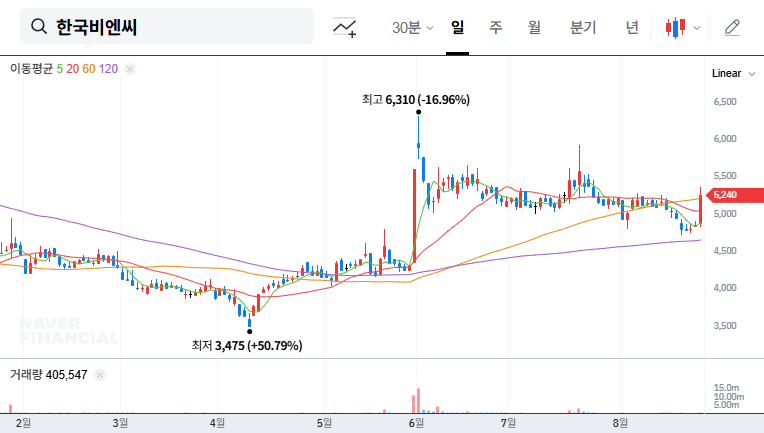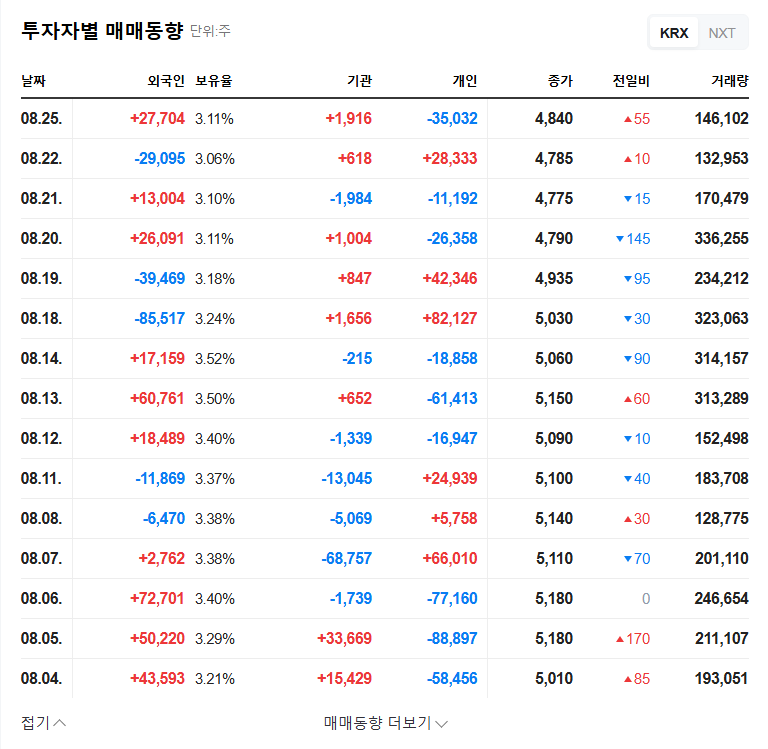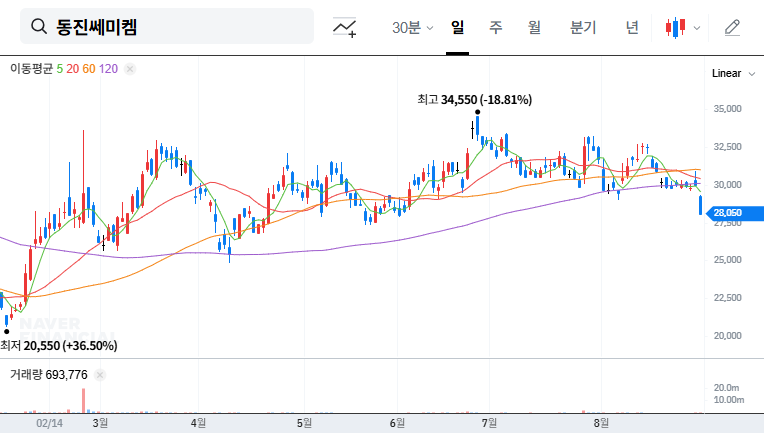
1. What Happened?
On September 1, 2025, Dongjin Semichem announced its decision to sell a portion (30%) of its stake in three Chinese subsidiaries (Beijing Dongjin Semichem, Sichuan Dongjin Electronic Materials, and Wuhan Dongjin Semichem) for KRW 62.1 billion. The expected date of the sale is May 31, 2026.
2. Why the Divestiture?
This divestiture is more than just recovering investment funds; it’s a strategic move to facilitate a joint venture related to display materials in China. The secured funds will be used to improve financial structure and invest in new businesses.
3. How Will This Divestiture Impact Dongjin Semichem?
Positive Impacts
- Improved Financial Structure: The inflow of KRW 62.1 billion will strengthen financial soundness, including reducing debt ratios.
- Investment in New Businesses: The secured funds will be used for investments to secure new growth engines.
- Strengthened Competitiveness in the Chinese Market: The joint venture will further solidify the company’s position in the Chinese market.
Negative Impacts and Considerations
- Potential for Disposal Losses: Losses may occur if the disposal price is lower than the book value.
- Exchange Rate Fluctuations: Actual disposal proceeds may vary due to exchange rate fluctuations.
4. What Should Investors Do?
This divestiture can be interpreted as a positive signal for Dongjin Semichem’s long-term growth. However, it’s crucial for investors to make informed decisions by continuously monitoring variables that may arise during the disposal process. Particular attention should be paid to exchange rate fluctuations and the possibility of disposal losses.
Frequently Asked Questions
Will Dongjin Semichem continue its operations in China after the divestiture?
Yes, as only a portion (30%) of the stake is being sold, Dongjin Semichem will maintain its influence in the Chinese market. Furthermore, it plans to strengthen its competitiveness within the Chinese market through the joint venture utilizing the funds from the divestiture.
How will the proceeds from the divestiture be used?
The proceeds will be used to improve financial structure and invest in new growth engines. Specific investment plans will be announced later.
Will this divestiture positively impact the stock price?
Generally, improving financial structure and securing new growth momentum have a positive influence on stock prices. However, continuous monitoring is necessary as stock price volatility can occur due to market conditions and other variables.