1. What Happened? Analysis of NKMAX’s Trading Halt Extension
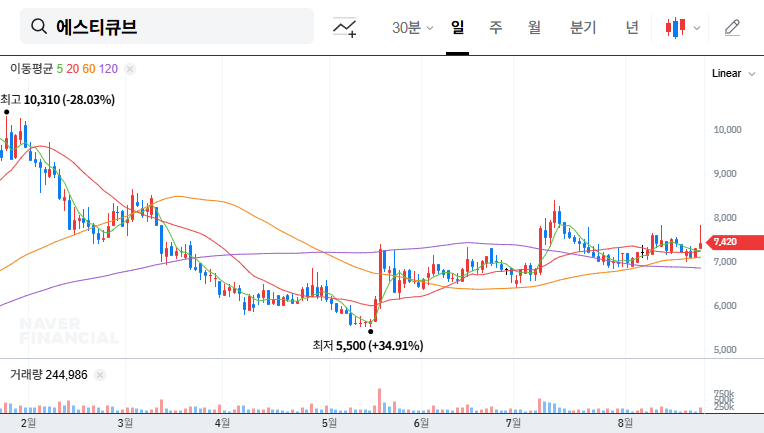
The Korea Exchange announced on September 3, 2025, a 15-business-day extension of the review period for determining whether NKMAX is subject to delisting review, until September 24th. This also means an extension of NKMAX’s trading halt.
2. Why Did This Happen? Background and Issues
NKMAX received approval for its rehabilitation plan in June 2025 and has been making efforts to normalize management, including changing its largest shareholder. However, there are still challenges to overcome, such as severe capital impairment and continuous operating losses. These factors influence the decision regarding delisting review.
- Positive Factors: Achieving rehabilitation milestones, change of largest shareholder, growth potential of the immune cell therapy market
- Negative Factors: Severe capital impairment, continuous operating losses, delisting uncertainty
3. What’s Next? Future Outlook and Scenarios
The extension of the review period can be interpreted as increasing the likelihood of delisting. NKMAX’s stock price is expected to be highly volatile depending on the final decision on September 24th. In the worst case, it could lead to delisting, causing significant losses to investors.
4. What Should Investors Do? Action Plan
Currently, investing in NKMAX carries very high risks. It is advisable to hold off on investments and observe the situation until the announcement on September 24th. If you have already invested, you should prepare for the possibility of delisting.
- Suspend investment and wait
- Consider the worst-case delisting scenario
- Strengthen information monitoring
Frequently Asked Questions
When will the results of NKMAX’s delisting review be announced?
It is scheduled to be announced on September 24, 2025.
What is the probability of NKMAX being delisted?
While the extension of the review period is interpreted as increasing the likelihood of delisting, nothing is confirmed. We must wait for the announcement on September 24th.
Should I sell NKMAX stock now?
Currently, trading is halted, so selling is not possible. The decision to sell should be made after the results are announced on September 24th, when trading resumes. Investment decisions should be made carefully, and consulting with a professional is recommended.