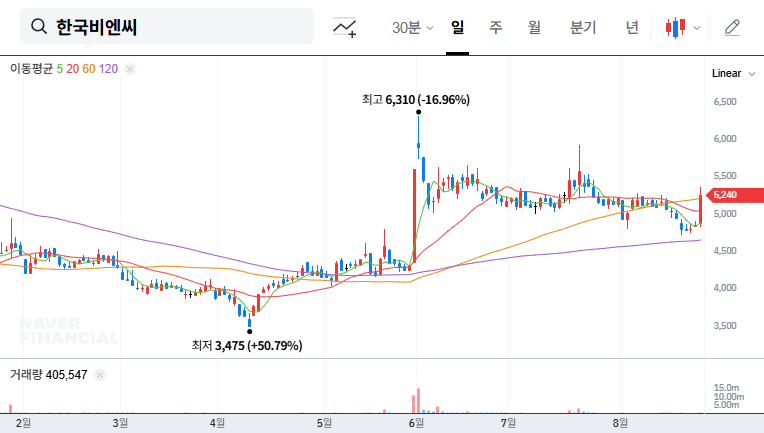
What Happened?
Korea BioNics received NMPA approval for two hyaluronic acid filler products, ‘CL-N’ and ‘N.’ Valid until August 21, 2030, these fillers are intended for the correction of moderate to severe nasolabial folds. Korea BioNics plans to commence sales through local distribution partners in China, alongside its existing ‘MAX’ product.
Why Does It Matter?
China is one of the fastest-growing beauty markets globally. This NMPA approval presents a significant opportunity for Korea BioNics, potentially leading to increased sales, portfolio diversification, and enhanced brand recognition. This approval is particularly significant as it marks the fruition of past R&D investments and could serve as a stepping stone for a turnaround.
What’s Next?
This approval paves the way for Korea BioNics to significantly expand its sales within the Chinese market. Its current low debt ratio and ample cash reserves provide a stable foundation for business expansion. However, potential risks, including cooperation with local partners, intensifying competition, and foreign exchange volatility, must be considered.
What Should Investors Do?
The NMPA approval is a positive sign, enhancing Korea BioNics’ long-term growth potential. However, investors should continuously monitor the competitive landscape in China, the company’s collaboration with local partners, and actual sales performance before making investment decisions.
Frequently Asked Questions
What is NMPA approval?
The NMPA (National Medical Products Administration) is China’s regulatory body for pharmaceuticals and medical devices. NMPA approval is mandatory for selling these products in China.
Why is this approval important for Korea BioNics?
China has a massive beauty market. This approval gives Korea BioNics a significant opportunity to enter the Chinese market, expand sales, and increase brand awareness.
What should investors be aware of?
Investors should monitor the competitive landscape in China, the relationship with local distribution partners, and actual sales performance. External factors like foreign exchange volatility should also be considered.

Leave a Reply