1. What Happened? ABL Bio Announces Positive ABL301 Phase 1 Results
ABL Bio announced positive results from its US FDA Phase 1 clinical trial for ABL301, a Parkinson’s disease treatment. The trial confirmed the safety and tolerability of the drug, with no serious adverse events related to the treatment and mostly mild reported side effects.
2. Why Does It Matter? Validation of Core Technology and Positive Investment Momentum
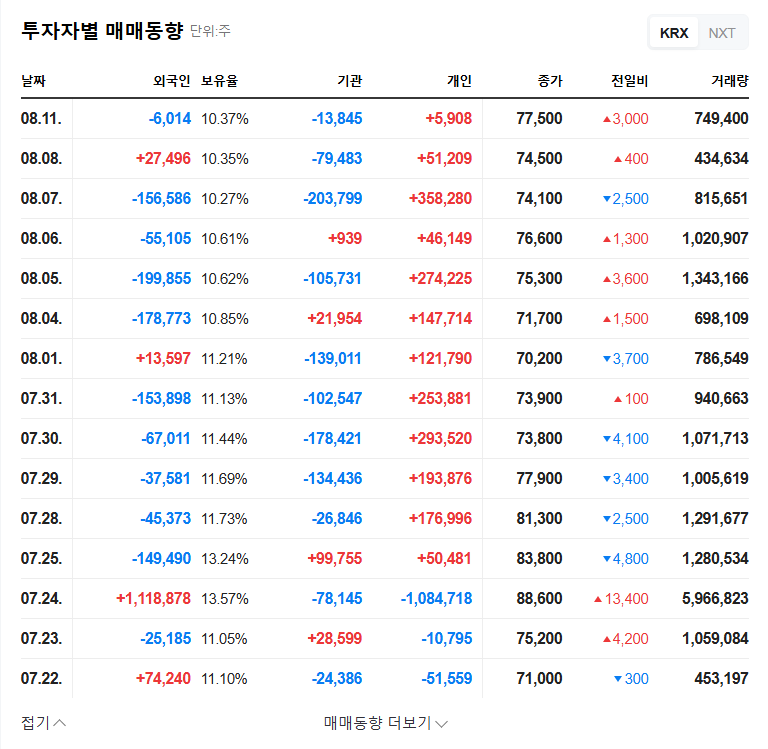
These Phase 1 results support the validity of ABL Bio’s core BBB shuttle platform technology and bolster confidence in its licensing agreement with Sanofi. Furthermore, the company’s 2025 semi-annual report indicates secured financial stability due to a large-scale licensing agreement.
3. What’s Next? Positive Outlook with Considerations
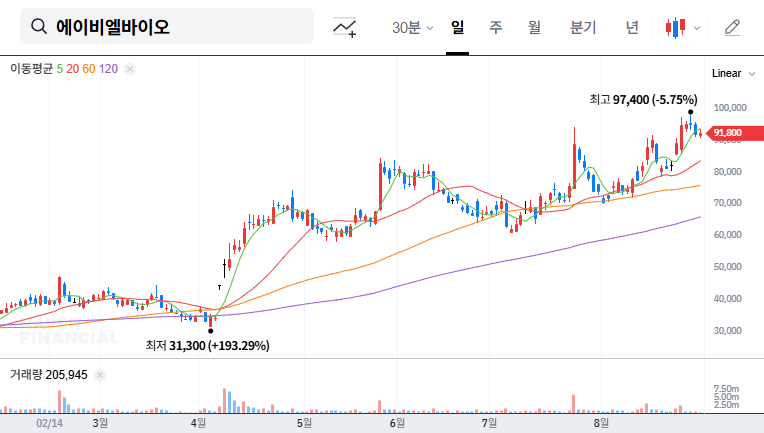
The success of ABL301’s Phase 1 trial is expected to positively influence investor sentiment. However, high R&D expenses and accumulated deficit remain risk factors. The successful execution of future clinical development and commercialization strategies will be crucial.
- Positive Factors: Successful ABL301 Phase 1 trial, large-scale licensing agreement, secured financial stability, strengthened ADC business
- Considerations: High R&D expenses and accumulated deficit, uncertainties in clinical development, intensifying competition
4. What Should Investors Do? Careful Investment Decisions After Reviewing Additional Information
Potential investors should carefully review additional information, such as detailed data from the ABL301 Phase 1 trial, licensing agreement terms, and future pipeline development plans, before making investment decisions. Continuous monitoring of macroeconomic conditions and the competitive landscape is also essential.
Why are the ABL301 Phase 1 results significant?
The confirmation of ABL301’s safety and tolerability in Phase 1 is a positive indicator of its potential as a Parkinson’s disease treatment. It also supports the validity of ABL Bio’s BBB shuttle platform technology and may positively influence the development of other pipelines.
What is ABL Bio’s financial status?
A large-scale licensing agreement has led to a surge in sales and improved financial structure. However, high R&D expenses and accumulated deficit remain risk factors to consider.
What are the key considerations for investment?
Uncertainties in clinical development, high R&D expenses, and intensifying competition should be considered. Reviewing additional information, such as detailed ABL301 Phase 1 data, licensing agreement terms, and future pipeline plans, is crucial before investing.