1. Onconik Therapeutics IR: What to Expect
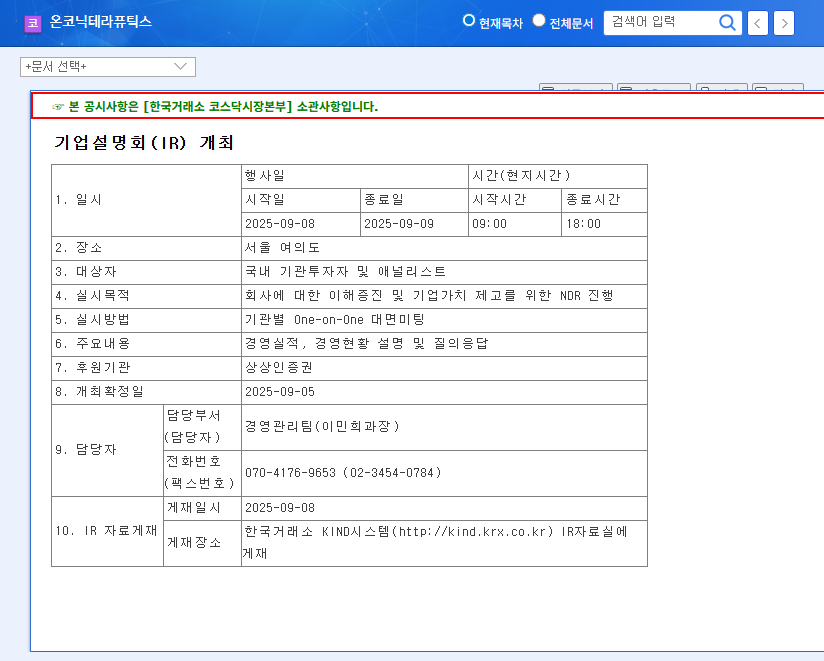
Onconik Therapeutics will hold an investor relations (IR) meeting on September 8th at 9:00 AM. This IR, part of a Non-Deal Roadshow (NDR), aims to enhance corporate value by providing updates on business performance, current status, and a Q&A session. Key information disclosures are expected, including Zastaprazan sales growth, Nesuparib clinical trial progress, and future business plans.
2. Why Pay Attention?: Growth Driver Analysis
- Zastaprazan Growth: The successful launch of Zastaprazan for erosive esophagitis and the additional approval for gastric ulcer treatment are expected to drive sales growth. Technology transfer agreements with China, India, and South America have also secured a foundation for royalty revenue.
- Nesuparib Clinical Expectations: Clinical development of the anticancer drug Nesuparib for pancreatic cancer, endometrial cancer, and other major cancers is progressing smoothly. Its designation as an orphan drug increases the likelihood of development and approval.
- Solid Financial Structure: Onconik successfully raised capital through its KOSDAQ listing and maintains a stable financial structure with KRW 45.1 billion in cash and cash equivalents.
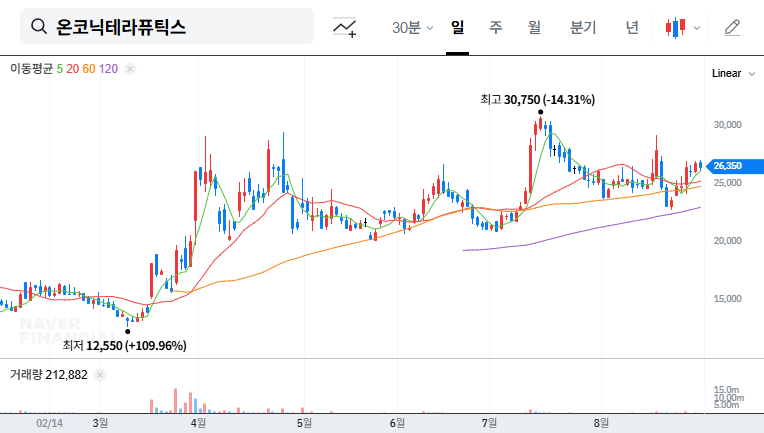
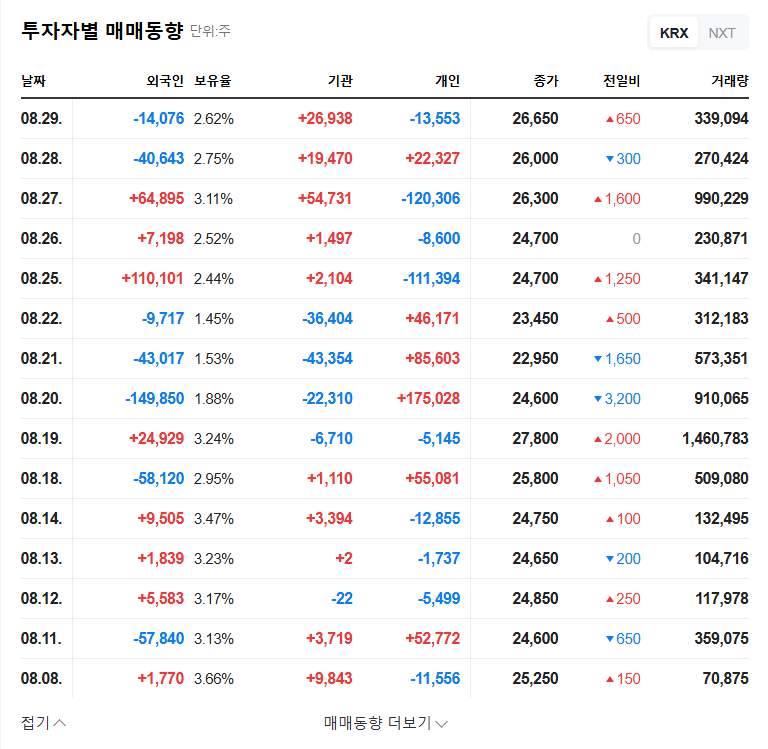
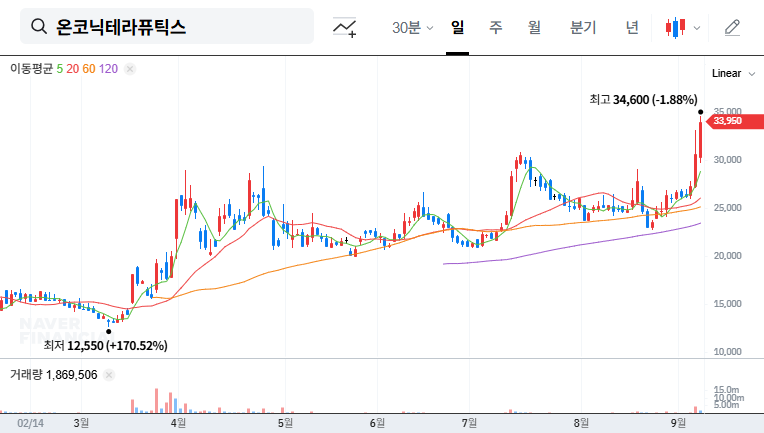
3. Post-IR Stock Outlook and Investment Strategy
Stock prices may fluctuate in the short term depending on the information disclosed at the IR. Positive information may create upward momentum, but failure to meet expectations could lead to selling pressure. In the mid-to-long term, Zastaprazan’s sales growth and the success of Nesuparib’s clinical trials will be key factors determining the stock’s trajectory. Investors should carefully analyze the IR content, future performance trends, and clinical results before making investment decisions.
4. Action Plan for Investors
If you are considering investing in Onconik Therapeutics, thoroughly review the information presented at the IR and the key points to watch. It is crucial to continuously monitor Zastaprazan sales, Nesuparib clinical results, and potential further technology transfer agreements. Changes in macroeconomic indicators, such as interest rates and exchange rates, should also be considered in your investment decision.
What are Onconik Therapeutics’ main business areas?
Onconik Therapeutics focuses on developing innovative new drugs for acid-related gastrointestinal diseases and cancer.
What conditions is Zastaprazan used to treat?
Zastaprazan is a P-CAB class drug used to treat erosive esophagitis and gastric ulcers.
What stage of clinical trials is Nesuparib currently in?
Nesuparib is currently undergoing clinical trials for several major cancers, including pancreatic and endometrial cancer, and has been designated as an orphan drug.
When is Onconik Therapeutics’ IR scheduled?
Onconik Therapeutics’ IR is scheduled for September 8, 2025, at 9:00 AM.